|
|
|
|

 PharmSchul
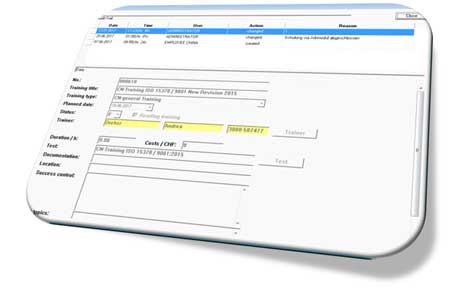
PharmSchul
Audit-Trail
All changes, made by PharmSchul users, will be documented by the additional module Audit-Trail.
The PharmSchul Audit-Trail meets the requirements of 21 CFR Part 11.
The following information will be stored in the Audit-Trail:
- Date of adaptation
-
Time of adaptation
-
User, who performed the adaptation
-
Type of adaptation: creation, modification, deletion
-
Reason of adaptation
-
Adaptations are highlighted
The reason for adaptation needs to be entered by changing or deleting a record.
By the PharmSchul User Management an easy traceability of changes made by PharmSchul users is ensured. The display of Audit-Trail entries is also managed by the user administration.
Of course the PharmSchul Audit-Trail is
validated.
|
|
|